Introduction to Calorimetry
Learning Outcomes
- Apply the safety rules in the chemistry laboratory through proper and safe handling of chemicals and chemical equipment.
- Identify and use common equipment and measuring devices in the chemistry laboratory.
- Properly record experimental data including the precision appropriate to the measuring devices used.
- Properly make measurements of length, mass, volume, and temperature.
- Properly perform the technique of filtration, quantitative transfer of materials, pipetting and use of the Bunsen burner.
- Tabulate and graph experimental data.
- Apply the steps of the scientific method.
- Determine energy changes in chemical reactions.
Specific heat capacity () is the amount of heat energy required to raise the temperature of 1 gram of a substance by 1°C (or 1 K). Each substance has its own unique specific heat value, which determines how quickly it heats up or cools down. For example, water has a relatively high specific heat of 4.18 J/g·°C, meaning it requires more energy to change its temperature compared to aluminum (0.89 J/g·°C) or copper (0.39 J/g·°C). In this experiment, you will determine specific heat using a coffee cup calorimeter, which consists of two nested Styrofoam cups with a lid to minimize heat loss. A thermometer or digital temperature probe will be used to measure initial and final temperatures of the water and metal, and a digital balance will measure the mass of both. A hot plate or boiling water bath will heat the metal to a known temperature, and tongs will safely transfer the hot metal to the calorimeter. Together, these tools allow for accurate measurement of heat transfer and calculation of specific heat capacity.
Coffee Cup Calorimeter Diagram
Here is a simple illustration of how your setup might look:
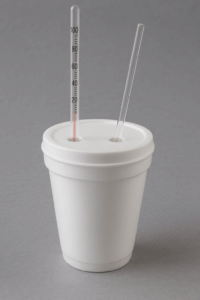
In this lab, we will make the simplifying assumption that no heat is lost to the surrounding environment. Under this assumption, the heat released by the hot metal cylinder when it is placed into cooler water is exactly equal to the heat absorbed by the water:
Since heat () can be calculated using the equation q = m × C × ΔT, we can substitute into the relationship above:
Rearranging this equation allows us to solve for the specific heat capacity of the metal:
Let’s walk through a sample data set to experimentally determine the specific heat of an unknown metal –
| Mass of metal | 100.0 g |
|---|---|
| Initial Temperature of metal | 100.0°C |
| Final temperature (metal & water) | 25.0°C |
| Mass of water | 150.0 g |
| Initial temperature of water | 20.0°C |
| Specific Heat of Water | 4.18 J/g·°C |
Step 1 – Calculate the change in temperature
For the metal:
For the water:
Step 2 – Use the specific heat equation
The calculated specific heat is 0.418 J/g·°C, which is very close to the accepted value for copper.
Once your experimental value is calculated, you can compare it to the accepted value using percent error:
Example: If the accepted value for copper is 0.390 J/g·°C:
This tells you how close your measurement is to the real value. A smaller percent error means your identification is likely correct. Your success depends on accurate measurements and careful handling of data—so take your time and double-check as you go!

