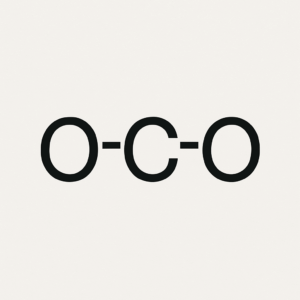
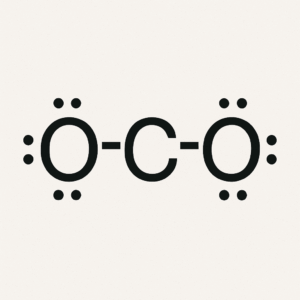
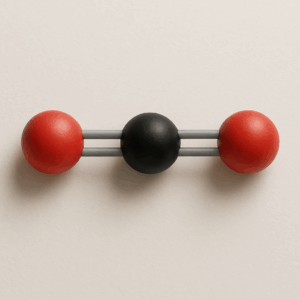
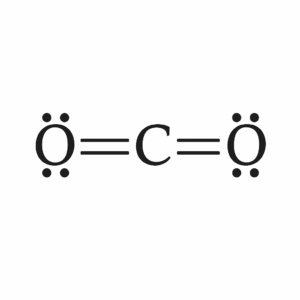We will focus on molecules and polyatomic ions that have single, double, or triple bonds and one central atom, which is the atom bonded to all the others. The main goal is to practice drawing Lewis structures—two-dimensional diagrams that show bonding and lone pairs of electrons—predicting electron geometry, which describes the arrangement of regions of electron density around the central atom, and predicting the molecular shape, which is the actual arrangement of atoms in space. You will also estimate bond angles; the angles formed between bonds originating from the same atom.
To accomplish this, we use the VSEPR Theory (Valence Shell Electron Pair Repulsion Theory), which states that electron regions repel each other and arrange themselves as far apart as possible. Key concepts include understanding a Lewis structure, which shows all the valence electrons in a molecule or ion; electron geometry, which considers both bonds and lone pairs; molecular shape, which focuses only on the atoms; and bond angles, which describe the spacing between bonds. Knowing molecular shape is critical because it directly impacts chemical behavior. For example, water’s bent shape makes it polar, giving it its remarkable ability to dissolve many substances, while carbon dioxide’s linear shape makes it nonpolar despite having polar bonds. Even biological processes like enzyme function rely on molecules fitting together with precise shapes, much like puzzle pieces. Whether in chemistry, medicine, engineering, or even cooking, understanding molecular structure is key, because shape controls function.